- Research
- 2022-2026 Research Strategy
- Open Clinical Trials
- Closed Clinical Trials
- What is a Clinical Trial?
- Why Participate in a Clinical Trial
- Remote Telehealth Pre-Screening Process
- Research Achievements
- Publications
- Research Development and Funding
- Participating Institutions
- International Collaboration
- BCT Trials & Projects Summary
- Translational Research
- Clinical Fellowship Program
- International Fellowship Support
- Annual Scientific Meeting
- Travel Grants and Awards
- About
- Our Impact
- Fundraise
- Donate
- Researcher Login
- Cart

0
INTERNATIONAL
Total number of trial
participants internationally
5
AUSTRALIA & NEW ZEALAND
Total number of trial participants
in Australia and New Zealand

15
INSTITUTIONS
Total number of participating institutions
in Australia and New Zealand
SORBET TRANSLATIONAL RESEARCH SAMPLES
SORBET PUBLICATIONS
2012
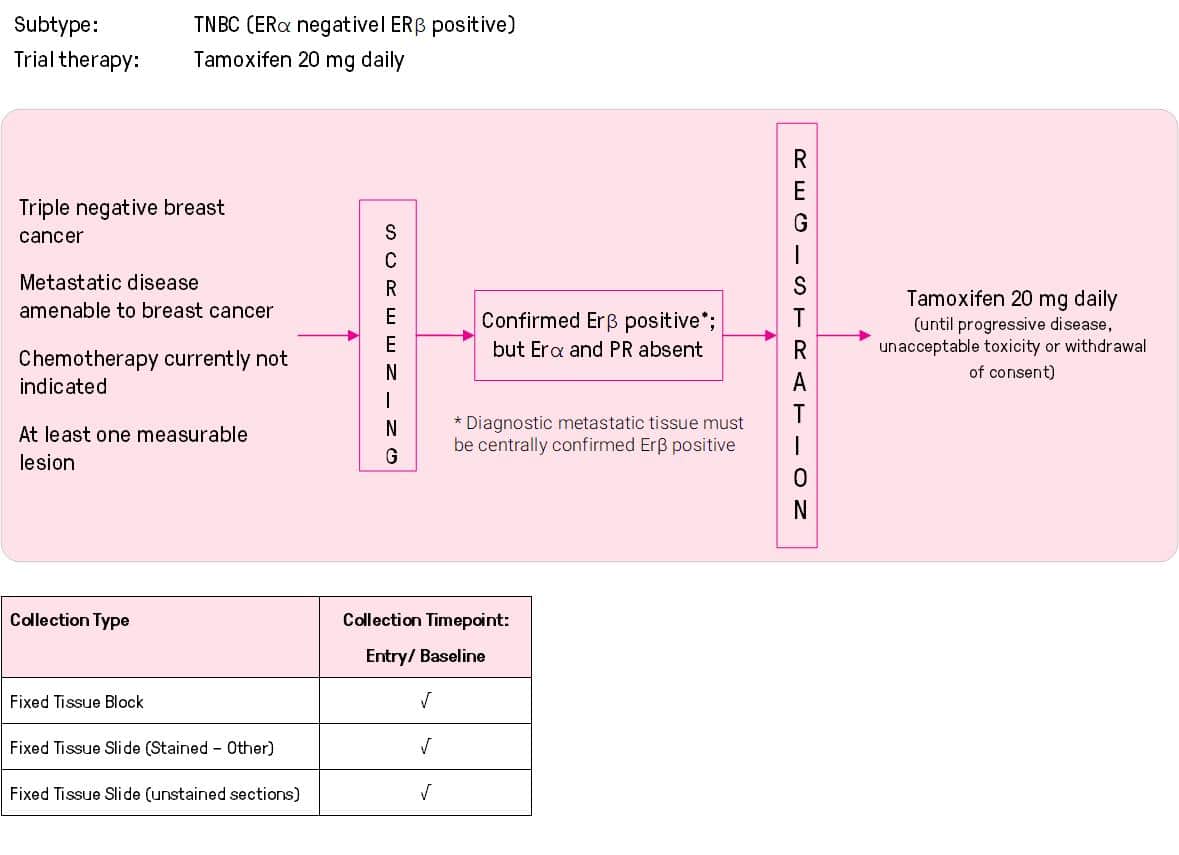
ANZ1001 SORBET: Study of oestrogen receptor beta and efficacy of tamoxifen, a single arm, phase II study of the efficacy of tamoxifen in triple negative but estrogen receptor beta positive metastatic breast cancer.
Phillips KA, Kiely BE, Francis PA, Boyle FM, Fox SB, Murphy L, Gebski V, Lindsay DF, Sutherland RL, Badger HD, Forbes JF. J. Clin Oncol. 2012; 30(Supp):TPS1136, Abstract
2011
ANZ 1001: SORBET, Study of Oestrogen Receptor Beta and Efficacy of Tamoxifen.
Kiely B, Phillips KA, Francis P, Boyle F, Forbes JF, Fox S, Murphy L, Gebski V, Lindsay DF, Sutherland R, Badger HD. ASCO 2011 2011; TPS126, Poster
2010
Clinical Trials Open for Participant Entry.
Forbes JF, Boyle F, Wilcken N, Lindsay DF, Leong E. COSA 2010; Poster 242, Poster
Institutions
-
NSW
-
SA
-
VIC
-
WA
-
New Zealand