- Research
- 2022-2026 Research Strategy
- Open Clinical Trials
- Closed Clinical Trials
- What is a Clinical Trial?
- Why Participate in a Clinical Trial
- Remote Telehealth Pre-Screening Process
- Research Achievements
- Publications
- Research Development and Funding
- Participating Institutions
- International Collaboration
- BCT Trials & Projects Summary
- Translational Research
- Clinical Fellowship Program
- International Fellowship Support
- Annual Scientific Meeting
- Travel Grants and Awards
- About
- Our Impact
- Fundraise
- Donate
- Researcher Login
- Cart
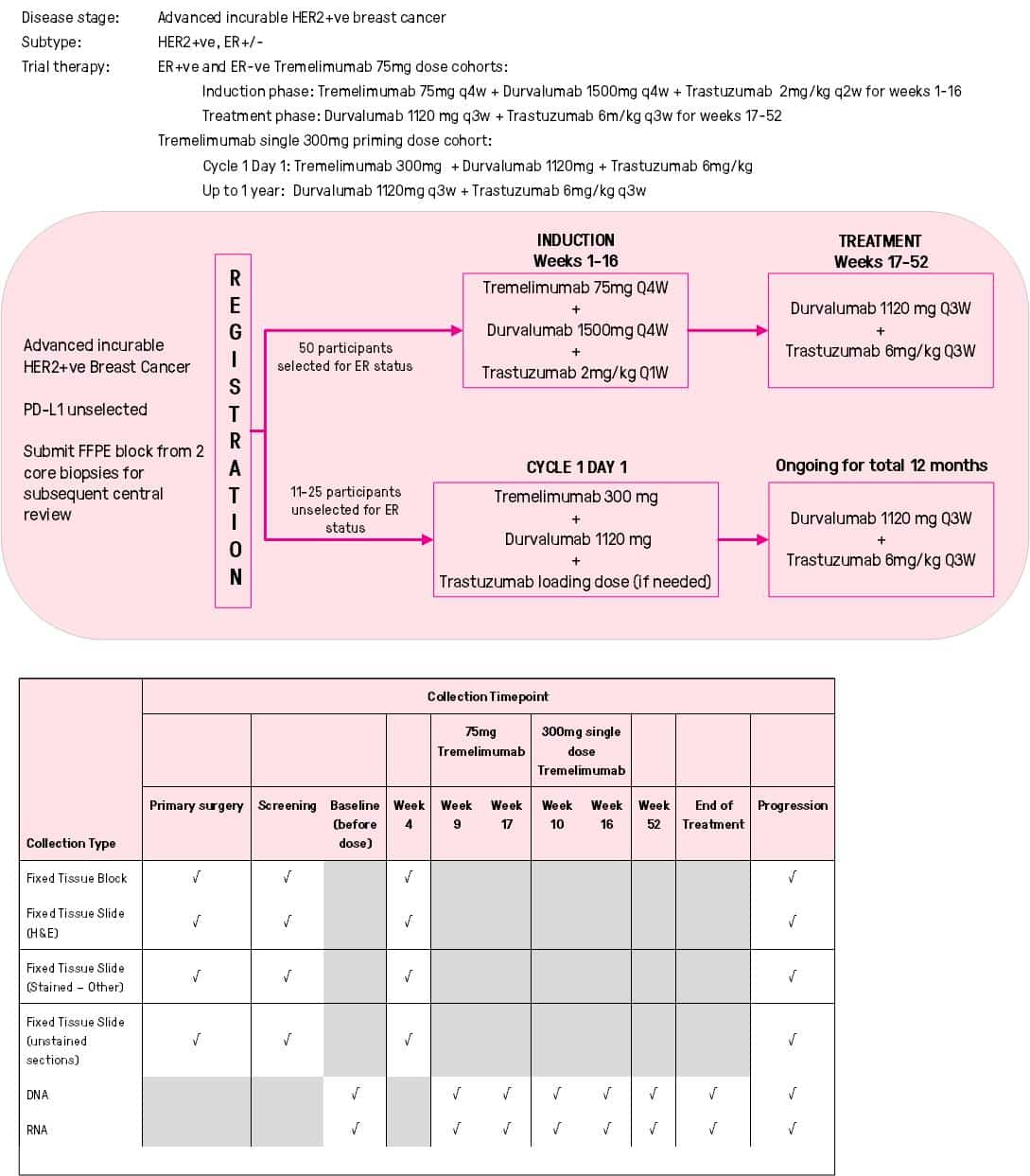
THE DIAmOND CLINICAL TRIAL
Researchers have found that sometimes the body’s own immune system may slow down or control cancer growth. But in some patients, cancer cells and immune cells start to send out signals that stop the body’s immune system from controlling the cancer.
The DIAmOND clinical trial will investigate the addition of two immunotherapy drugs to Herceptin (Trastuzumab) as treatment for women and men who have HER2-positive breast cancer which has spread beyond the breast.
New immunotherapy drugs like Durvalumab and Tremelimumab assist the body’s natural immune system to attack the cancer cells. The combination of these two drugs has been given previously to people with lung cancer, but this is the first trial that will test the combination of these two drugs in people with breast cancer.
It is hoped that by combining Durvalumab and Tremelimumab with Herceptin in patients with advanced HER2-positive breast cancer, it will stop or slow down the growth of the cancer, prolong lives as well as result in excellent quality of life.
DIAmOND is an Australian clinical trial and will include 50 participants and involve 10 hospitals in Australia.