- Research
- 2022-2026 Research Strategy
- Open Clinical Trials
- Closed Clinical Trials
- What is a Clinical Trial?
- Why Participate in a Clinical Trial
- Remote Telehealth Pre-Screening Process
- Research Achievements
- Publications
- Research Development and Funding
- Participating Institutions
- International Collaboration
- BCT Trials & Projects Summary
- Translational Research
- Clinical Fellowship Program
- International Fellowship Support
- Annual Scientific Meeting
- Travel Grants and Awards
- About
- Our Impact
- Fundraise
- Donate
- Researcher Login
- Cart
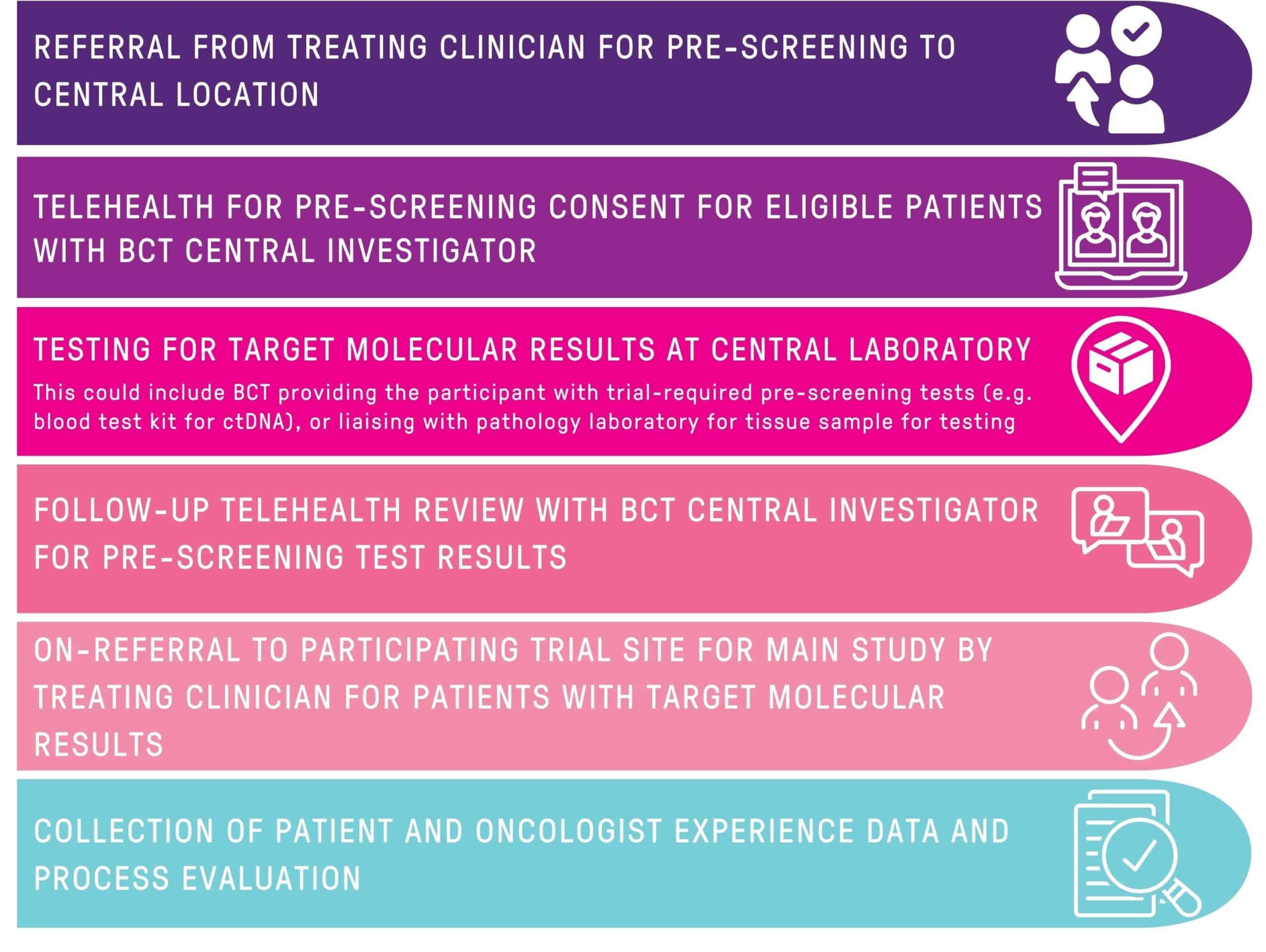
BCT is trialing a remote telehealth pre-screening process to broaden clinical trials access to regional and remote patients and improve clinical trial recruitment.
The focus for this process is on trials with molecular targets; that is, where the cancer being treated in the trial needs to have specific characteristics (e.g. a particular mutation; an enzyme that is present or absent), which is identified by looking at a blood sample or a sample of the cancer.
Patients with potential eligibility for a particular trial will be referred to BCT’s Central Investigator by their usual oncologist. They will have a telehealth consultation, where the trial will be explained, their questions answered, and they will be asked to consent to the pre-screening procedure.
BCT can provide participants with a trial-required test e.g. blood test kit for circulating tumour DNA (ctDNA) or liaison with the pathology laboratory for a tissue sample for testing.
Samples will be tested for the trial-specific molecular target at a central laboratory. The patient will then have a follow-up telehealth review with the BCT Central Investigator to inform them of their results, and if they are eligible for the main part of the clinical trial.
Care will continue with their treating oncologist during this process. Their oncologist will refer them to a centre that is participating in the main part of the clinical trial for further discussions with the study team and potentially consent to take part in the main part of the clinical trial.
The process aims to give much-needed clinical trial access to rural, regional, and remote patients, allowing them to complete pre-screening in their local community; as well as increase the reach of trials targeting niche patient cohorts with particular molecular profiles. Remote pre-screening is being used for the OLIO clinical trial.
Aims:
- Develop a process for remote pre-screening for molecularly targeted clinical trials via telehealth
- Implement the remote pre-screening process at Pilot site/s and Australia-wide
- Review the remote pre-screening process outcomes
- Assess patient and clinician experiences to improve the remote pre-screening process.
We hope this project will have a range of other benefits, including:
- Improving the diversity of BCT trial participants
- Improving the cancer outcomes for both trial and non-trial patients at involved sites
- Improving the robustness of BCT trial results; and
- Supporting the research capacity of non-BCT sites.
We also hope our experience may be useful in a non-cancer trial context.