- Research
- 2022-2026 Research Strategy
- Open Clinical Trials
- Closed Clinical Trials
- What is a Clinical Trial?
- Why Participate in a Clinical Trial
- Remote Telehealth Pre-Screening Process
- Research Achievements
- Publications
- Research Development and Funding
- Participating Institutions
- International Collaboration
- BCT Trials & Projects Summary
- Translational Research
- Clinical Fellowship Program
- International Fellowship Support
- Annual Scientific Meeting
- Travel Grants and Awards
- About
- Our Impact
- Fundraise
- Donate
- Researcher Login
- Cart
WHO CAN TAKE PART IN CAMBRIA-2?
CAMBRIA-2 is for men or women who have ER-positive, HER2-negative early breast cancer that has an intermediate or high risk of coming back (recurrence).
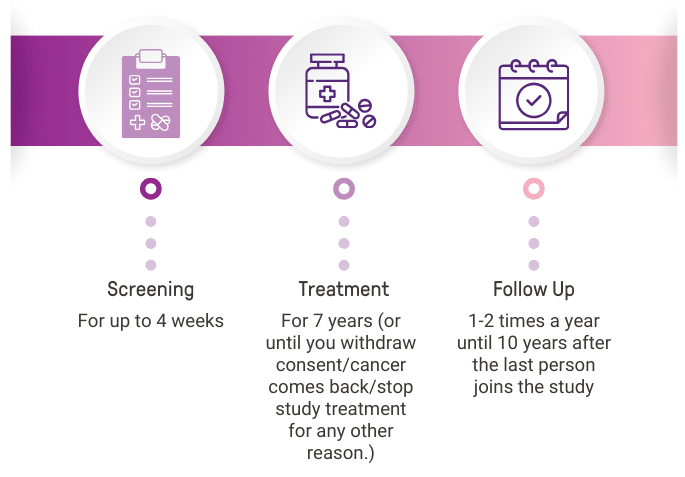
WHAT DOES TAKING PART IN CAMBRIA-2 INVOLVE?
Participants may be involved in this study for 10-13 years.

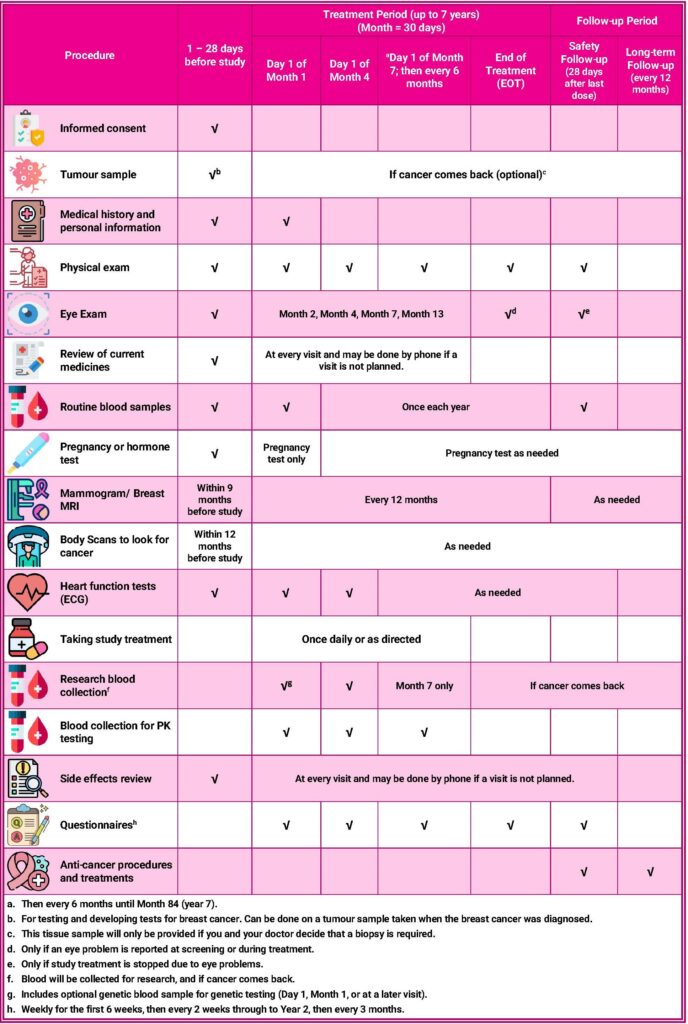
CAMBRIA-2 Trial Procedures
CAMBRIA-2 – optional parts of the study
There are a couple of procedures that are optional parts of CAMBRIA-2. Optional means that you can still take part in CAMBRIA-2, whether you agree to taking part in one, both, or none of these procedures. You will specifically document that you choose whether or not you want to take part in these procedures.
Genetic Testing
If you consent (agree) to Genetic testing, you will have an additional 6 mL blood sample taken during the study. Genetic research can help find new ways to detect, treat, prevent or cure health problems, such as breast cancer. Your sample will be tested at laboratories in the USA and UK, and will be stored in the UK for up to 15 years after the study ends, and will then be destroyed. You will not receive the results of the testing, and only summary results (not your individual results) will be used by researchers for medical and healthcare research.
Tissue Banking
Tissue Banking is a common part of many clinical trials, including CAMBRIA-2. Tissue banking is donating any tumour tissue or blood samples left over after testing for CAMBRIA-2 to a specific Tissue Bank for use in future research. The samples in CAMBRIA-2 will be stored at a Tissue Bank in the UK. The Tissue Bank must ensure proper standards are met in storing and managing your samples. Any future new studies that use your samples must have appropriate approvals in place (e.g. from a Human Research Ethics Committee) before your samples can be used.
IF YOU THINK THE CAMBRIA-2 TRIAL MIGHT BE RIGHT FOR YOU …
Please speak to your regular doctor or oncologist about a referral to a participating site.
You can give your doctor/oncologist the link to the trial page for further information.